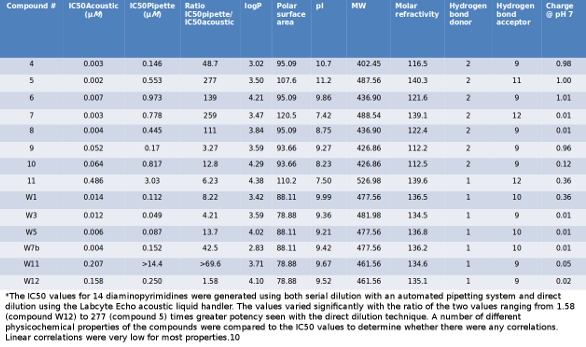
With filter, and analysed by TEMPO and standard methods in parallel. Standard method protocol: 1 ml of primary dilution and serial dilutions up to 10-3 in Peptone salt were included in appropriate agar, 3 dilutions, 2 plates per dilution.After incubation (Table 3), colonies were counted by visual inspection. Automated method protocol. A serial dilution is the stepwise dilution of a substance in solution.Usually the dilution factor at each step is constant, resulting in a geometric progression of the concentration in a logarithmic fashion. A ten-fold serial dilution could be 1 M, 0.1 M, 0.01 M, 0.001 M.Serial dilutions are used to accurately create highly diluted solutions as well as solutions for experiments resulting in. Serial Vs Parallel Dilution Method. See also SIMPLE DILUTION 2. There are two problems with this series of. Many laboratory protocols require the serial dilution of reagents or compounds. If the dilution. 3 uL = 297 uL diluent to perform the 1:100 dilution.
Click to see full answer.
Consequently, what are the advantages of serial dilution?
Serial dilution has many advantages: the materials necessary are typically already present in the lab and require no special engineering. Conditions can be adjusted as the experiment progresses (e.g., drug concentrations increased as drug resistance improves).

With filter, and analysed by TEMPO and standard methods in parallel. Standard method protocol: 1 ml of primary dilution and serial dilutions up to 10-3 in Peptone salt were included in appropriate agar, 3 dilutions, 2 plates per dilution.After incubation (Table 3), colonies were counted by visual inspection. Automated method protocol. A serial dilution is the stepwise dilution of a substance in solution.Usually the dilution factor at each step is constant, resulting in a geometric progression of the concentration in a logarithmic fashion. A ten-fold serial dilution could be 1 M, 0.1 M, 0.01 M, 0.001 M.Serial dilutions are used to accurately create highly diluted solutions as well as solutions for experiments resulting in. Serial Vs Parallel Dilution Method. See also SIMPLE DILUTION 2. There are two problems with this series of. Many laboratory protocols require the serial dilution of reagents or compounds. If the dilution. 3 uL = 297 uL diluent to perform the 1:100 dilution.
Click to see full answer.
Consequently, what are the advantages of serial dilution?
Serial dilution has many advantages: the materials necessary are typically already present in the lab and require no special engineering. Conditions can be adjusted as the experiment progresses (e.g., drug concentrations increased as drug resistance improves).
Furthermore, what is serial dilution and why is it used? Serial dilution is the stepwise dilution of a substance in solution. Serial dilutions are used to accurately create highly diluted solutions as well as solutions for experiments resulting in concentration curves with a logarithmic scale.
Secondly, is direct or serial dilution more accurate?
The direct dilution method uses far less sample than the serial dilution method. This figure shows only the first four concentrations via direct dilution. Essential to direct dilution is the ability to accurately transfer extremely small volumes of stock solution, which is generally not possible with pipets.
What is the advantage of performing a serial dilution instead of a single dilution?
Easier and Faster Preparation of Calibration StandardsThe errors introduced with each successive dilution drops proportionately with the solution concentration. Preparing a series of calibration standards by this method reduces the amount of required time.
This section is not a recipe for your experiment. It explains someprinciples for designing dilutions that give optimal results. Onceyou understand these principles, you will be better able to designthe dilutions you need for each specific case.
Often in experimental work, you need to cover a range ofconcentrations, so you need to make a bunch of differentdilutions. For example, you need to do such dilutions of thestandard IgG to make the standard curve in ELISA, and then againfor the unknown samples in ELISA.
You might think it would be good to dilute 1/2, 1/3, 1/10, 1/100.These seem like nice numbers. There are two problems with this series ofdilutions.
- The dilutions are unnecessarily complicated to make. You need to do a differentcalculation, and measure different volumes, for each one. It takes a longtime, and it is too easy to make a mistake.
- The dilutions cover the range from 1/2 to 1/100 unevenly.In fact, the 1/2 vs. 1/3 dilutions differ by only 1.5-fold in concentration,while the 1/10 vs. 1/100 dilutions differ by ten-fold. If you are going tomeasure results for four dilutions, it is a waste of time and materialsto make two of them almost the same. And what if the half-maximal signaloccurs between 1/10 and 1/100? You won't be able to tell exactly where itis because of the big space between those two.
Serial dilutions are much easier to make and they cover the range evenly.
Serial dilutions are made by making the same dilution step over and over,using the previous dilution as the input to the next dilution in each step.Since the dilution-fold is the same in each step, the dilutionsare a geometric series (constant ratio between any adjacent dilutions).For example:
- 1/3, 1/9, 1/27, 1/81
1 500 Serial Dilution
Notice that each dilution is three-fold relative to the previous one.In four dilutions, we have covered a range of 181/3 = 60-fold.If that isn't enough range, consider a series of five-fold dilutions:- 1/5, 1/25, 1/125, 1/625
When you need to cover several factors of ten (several 'orders of magnitude') witha series of dilutions, it usually makes the most sense to plot the dilutions(relative concentrations) on a logarithmic scale. This avoids bunching mostof the points up at one end and having just the last point way fardown the scale.
Before making serial dilutions, you need to make rough estimatesof the concentrationsin your unknowns, and your uncertainty in those estimates. For example,if A280 says you have 7.0 mg total protein/ml, and you thinkthe protein could be anywhere between 10% and 100% pure, then yourassay needs to be able to see anything between 0.7 and 7 mg/ml.That means you need to cover a ten-fold range of dilutions, or maybe a bitmore to be sure.
If the half-max of your assay occurs atabout 0.5mg/ml,then your minimum dilution fold is(700mg/ml)/(0.5mg/ml) = 1,400.Your maximum is(7000mg/ml)/(0.5mg/ml) = 14,000.So to be safe, you might want to cover 1,000 through 20,000.
In general, before designing a dilution series, you need to decide:
- What are the lowest and highest concentrations (or dilutions)you need to test in order to be certain of finding the half-max? Thesedetermine the range of the dilution series.
- How many tests do you want to make? This determines the size of theexperiment, and how much of your reagents you consume. More tests will coverthe range in more detail, but may take too long to perform (or cost too much).Fewer tests are easier to do, but may not cover the range in enough detailto get an accurate result.
- What volume of each dilution do you need to make in order to haveenough for the replicate tests you plan to do?
Serial Dilution Procedure
Now suppose you decide that six tests will be adequate (perhapseach in quadruplicate).Well, starting at 1/1,000, you need five equal dilution steps (giving yousix total dilutions counting the starting 1/1,000) that end ina 20-fold higher dilution (giving 1/20,000). You can decide on a goodstep size easily by trial and error. Would 2-fold work? 1/2, 1/4, 1/8, 1/16, 1/32. Yes, in factthat covers 32-fold, more than the 20-fold range we need. (The exact answeris the 5th root of 20, which your calculator will tell you is 1.82 foldper step. It is much easier to go with 2-fold dilutions and gives about thesame result.)
So, you need to make a 1/1,000 dilution to start with. Then you need toserially dilute that 2-fold per step in five steps. You could make 1/1,000 byadding 1 microliter of sample to 0.999 ml diluent. Why is that a poor choice?Because you can't measure 1 microliter (or even 10 microliters) accuratelywith ordinary pipeters. So, make three serial 1/10 dilutions(0.1 ml [100 microliters] into 0.9 ml): 1/10 x 1/10 x 1/10 = 1/1,000.
Serial Dilution Calculator
Now you could add 1.0 ml of the starting 1/1,000 dilution to1.0 ml of diluent, making a 2-fold dilution (giving 1/2,000).Then remove 1.0 ml from that dilution (leaving 1.0 ml for yourtests), and add it to 1.0 ml of diluent in the next tube (giving1/4,000). And so forth for 3 more serial dilution steps (giving1/8,000, 1/16,000, and 1/32,000). You end up with 1.0 ml of each dilution.If that is enough to perform all of your tests, this dilution planwill work. If you need larger volumes, increase the volumes you useto make your dilutions (e.g. 2.0 ml + 2.0 ml in each step).
